碱性试剂:
1)氨基钠
适合于苯环上没有电负性取代基,否则易发生birch还原反应。
2)N-甲基苯基氨基钠/HMPT
对于苯环上有电负性取代基的底物易发生取代反应。

Typical Procedure. Cleavage of 1,2,4-Trimethoxybenzene. 17 N-Methylaniline (2.68 g, 25 mmol) is added dropwise at65 °Cto a stirred suspension of sodium hydride (0.6 g, 25 mmol) in sodium-dried xylene (5 mL) and HMPT (4.26 g, 25 mmol, distilled over calcium hydride and stored in dark over molecular sieves,8A°). After 15 min, the ether (12.5 mmol in the minimum amount of xylene) is added and the mixture is heated at85°C. The reaction is monitored by GLC (3 mSE 30 column) and by TLC. When the starting material disappears (6 hrs) the mixture is poured into water, acidified with dilute hydrochloric acid, and the product is extracted with ether. The ether phase is washed with dilute hydrochloric acid (2×90 mL) to remove HMPT and the amines and then the product is extracted with 10% sodium hydroxide solution (2×90 mL). The aqueous phase is acidified with dilute hydrochloric acid and extracted with ether (3×90 mL). The organic phase is dried over calcium chloride and concentrated on a rotary evaporator to give pure 2,5-dimethoxyphenol; yield:1.9 g(90%). The product may be further purified by column chromatography on silica gel.
3.3)EtSNa
在EtSNa/DMF体系中,双键和溴代物不受影响;有时具有选择性去甲基化,如下表。
范例:
Table 8. Dealkylation of Ethers by Sodium Ethanethiolate
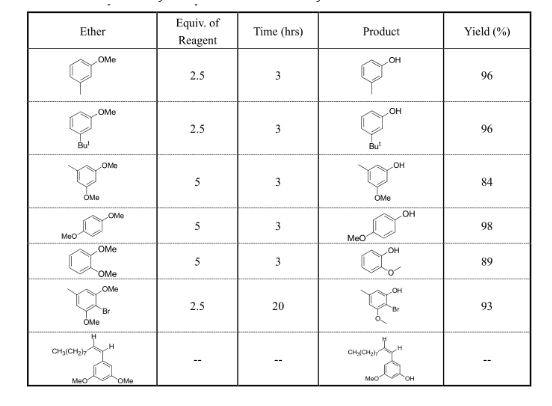
Typical Procedure. Dealkylation of m-Methoxytoluene.18Ethanethiol (1.25 g, 11.8 mmol) dissolved in dry DMF (20 mL) is added to a suspension of sodium hydride (1.0 gof a 50% oil dispersion) in dry DMF (10 mL) under an atmosphere of nitrogen. The mixture is stirred for 5 min before solution of m-methoxytoluene (1.09 g) in dry DMF is added. The solution is then refluxed for 3 hrs. The cooled mixture is acidified with 10% aqueous hydrochloric acid and extracted with ether. The ether layer is washed with water and extracted with 5% sodium hydroxide. The alkaline extracts are then acidified and reextracted with ether. The ethereal solution is washed with water, dried and evaporated to give m-cresol as a light brown oil; yield:0.85 g(78%).
其它试剂
1)Me3SiI
另一种常用的去甲基试剂,在此体系中,双键、三键、酮羰基、氨基和卤代物稳定,但苄醚、三苯甲基醚、叔丁基醚不稳定。三甲基碘硅烷能裂解酯但比醚慢。
范例:
Table 10. Dealkylation of Ethers byTrimethylsilyl Iodide

General Procedure forDealkylation of Ether. 19 To a 2 Msolution of ether (1 equiv.) in a suitable solvent (Table 10) is added neattrimethylsilyl iodide (1.3 equiv.) through a dry syringe. The reaction ismaintained at temperature indicated in Table 10 and monitored by NMR forcompletion. Yields are calculated by NMR integration of pertinent peaks. Forisolation of the alcohols, at the completion of the reaction, the excesstrimethylsilyl iodide is destroyed and the intermediate trimethylsilyl etherformed during the reaction is hydrolyzed to alcohol by pouring the reactionmixture into methanol (4 equiv.). The volatile components are removed atreduced pressure and the residue is taken up in ether, washed with aqueoussodium bisulfite, aqueous sodium bicarbonate and brine and dried. The residueleft after evaporation of solvent is further purified (if necessary) by columnchromatography on silica gel.
声明:
本文非原创内容,版权和内容解释权归原作者所有。在这里仅供交流学习使用,若涉及版权问题,请联系删除。