


Home

Catalog
Search
|
4-(2-羟乙基)-2,2-二甲基-1,3-二氧戊环正在热销,欢迎询价
别名:4-(2-羟乙基)-2,2-二甲基-1,3-二氧戊环
英文名称:4-(2-Hydroxyethyl)-2,2-dimethyl-1,3-dioxolane
分子式:C7H14O3
Cas No.:5754-34-7
分子量:146.18
结构式: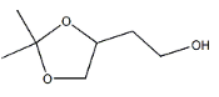 规格:97% 外观:无色液体 用途:基础原料、有机中间体、医药中间体 合成路线如下: toluene-4-sulfonic acid; sodium hydrogencarbonate; acetone; Reactants are commercially availlable.
MERCK FROSST CANADA and CO., WO2005/70874, A1, (2005) EXAMPLE 4 3,4-bis (nitrooxy) butyl (2Z)-4- (acetyloxy)-3- [4- (methylsulfonyl) phenyl]-2-phenylbut-2-enoate Step 1: 3,4-bis (nitrooxy) butyl alcohol To a solution of 27.8 g of butan-1, 2,4-triol in 150 mL of acetone was added 27.3 mL of 2,2 - dimethoxypropane and 10 mg of PTSA at RT. The reaction was stirred for 3h then quenched by addition of 200 mg of NaHCO3. After stirring for 15 min, the solvent was evaporated. The crude compound was filtered through a pad of silica gel us ing 60percent EtOAc/hexanes to afford 30 g of the desired compound as a colorless oil. The compound was used directly in the next step. To a solution of 20 g of 2-(2, 2-dimethyl-1, 3-dioxolan-4-yl) ethanol in 200 mL of CH2Cl2 at 0 C were ad ded 33.5 g of DMAP and 26 mL of acetic anhydride. The mixture was stirred 2 h at RT then quenched by filtration through a short pad of silica gel. Purification by flash chromatography afforded 20.7 g of the desired compound as a colorless o il. The compound was used directly for the next step. Sulfuric acid (40 mL) was added to nitric acid (20 mL) at-78 C and then stirred at 0 C for 10 min. At-78 C, a solution of 10 g of 2-(2, 2-dimethyl-1, 3-dioxolan- 4-yl) ethyl acetate in 2 0 mL of CHzClz was added and the reaction was stirred for 1 h. Quenching was done by adding the reaction mixture to 500 g of ice in an 1 L erlenmeyer flask. The compound was then extracted with CH2Cl2, washed with NaHC03, brine and dried ov er sodium sulfate. The crude compound was used directly for the next step without further purification. To a solution of 5.1 g of 3,4-bis (nitrooxy) butyl acetate in 40 mL of EtOH at 25 C was added 855 mg of NaOH. The mixture was stirred fo r 1 h, quenched by addition of NaHC03 sat. , extracted with EtOAc, washed with brine and dried over sodium sulfate. Purification by flash chromatography afforded lg of the desired compound as a yellowish oil.'H NMR (acetone-d6, 500 MHz) : 8 5.71-5. 66 (m, 1H), 5.07 (dd, 1H), 4.76 (dd, 1H), 3.97 (t, 1H), 3.77-3. 68 (m, 2H), 2.02-1. 95 (m, 2H).
|
|