别名:3-甲基-2-氧代丁酰乙酯
英文名称:Ethyl 3-methyl-2-oxobutyrate
分子式:C7H12O3
Cas No.:20201-24-5
分子量:144.17
结构式: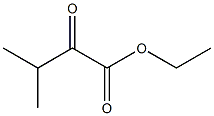
规格:95%
外观:黄色液体
用途:基础原料、有机中间体、医药中间体
合成工艺如下:
water; 20 wt percent Ba on decomposed (M2+1-x M3+1-x(OH)2)(An?x/n).yH2O, where M2+=Zn and Mg, M3+=Al, x=0.382
Reactants are commercially availlable.
Kourtakis, Kostantinos, US2006/84818, A1, (2006) EXAMPLES; 20 wt percent Ba on decomposed (M2+1-x M3+1-x(OH)2)(An-x/n).yH2O, where M2+=Zn and Mg, M3+=Al, x=0.382 Catalyst 1Barium acetate (Aldrich, Milwaukee Wis.) was dissolved in approximately 40 ml water and was allowed to contact approximately 10 g of Hydrotalcite Extrudates (Sud-Chemie). The hydrotalcite has an atomic ratio Zn 0.16 Mg 0.46 Al 0.382. The resulting material was allowed to dr y at room temperature and was loaded into a 1 diameter fritted quartz tube. The material was heated in a vertical tube furnace according to the following schedule: (All process steps were performed in at least 100 standard cubic centimeters per minute of flowing air) (i) heat to 120°C., and hold at 120°C.; heat at a rate of 5°C./minute to 300°C., and hold for five hours at 300°C.; cool to roo m temperature. Decomposed (M2+1-xM3+x(OH)2)(An-x/n).yH2O, where M2+=Mg, M3+=Al, x=0.25 Catalyst 2 In a one liter round bottom flask, 51.28 g of magnesium nitrate hexahydate, Mg(NO3)2.6H2O (EM Science s) and 25.01 g of aluminum nitrate (EM Sciences) were dissolved in approximately 500 ml of water. Th e solution was heated to 60°C. to 70°C. Approximately 140 ml of 30 wt percent ammonium hydroxide w as slowly added to the stirred solution over a period of about 1 hour. After stirring for another 30 minutes at 60°C., the mixture was allowed to cool to room temperature. The material, which was a c loudy precipitate, was dried overnight at room temperature, in flowing nitrogen, before heating. The dried material was loaded into an alumina boat and heated in a horizontal tube furnace. The airflow rate corresponded to a linear velocity of 15.6 cm/min. The material was heated at a rate of 5°C./m in to 120°C.; this temperature (120°C.) was maintained for four hours. It was subsequently heated at a rate of 5°C./min to approximately 450°C. and then allowed to cool to room temperature in flow ing air. Decomposed (M2+1-xM3+x(OH)2)(An-x/n).yH2O, where M2+=Mg, M3+=Al, x=0.25 Catalyst 3 A simil ar procedure as described for catalyst 2 was used; however, a more dilute aqueous combined solution of magnesium and aluminum nitrate was employed. 10.26 grams of Mg(NO3)2.6H2O was dissolved in 400 ml of water in a 1 liter round bottom flask. To this solution, 5 g of aluminum nitrate dissolved in 10 ml of water was added. The solution was stirred, and the temperature was raised to 60°C. to 70°C. About 50 milliters of 30 wt percent NH4OH was added to this solution over a period of 1 hour. The solution was stirred for another 30 minutes and then allowed to cool over a two hour time period (whi le stirring) to room temperature. The precipitate was dried under nitrogen for about 12 hours. The dried material was loaded into an alumina boat and heated in a tube furnace. The airflow rate corresp onded to a linear velocity of 15.6 cm/min. The material was heated at a rate of 5°C./min to 120°C. ; this temperature (120°C.) was maintained for four hours. It was subsequently heated at a rate of 5°C./min to approximately 450°C. and then allowed to cool to room temperature in flowing air. Exam ple of Vapor Phase Reaction Solutions containing gamma-valerolactone in formalin (37percent aqueous formaldehyde), at various feed ratios, were fed to a vaporizer (held at 200°C.) followed by the int roduction of nitrogen, to carry the vapor through a 1/4 inch tubular reactor containing a catalyst h eated to the appropriate reaction temperature. In the following examples, nitrogen flow rate was 24 cc/min., liquid feed rate was 1 ml/hr, formaldehyde to GVL molar ratio was 4:1 and the catalyst volu me was 2 cc. The TOS (hours) was typically 0.5 to 5 hours. The reactor effluent was condensed in a cold trap and analyzed off-line by GC-MS using an internal standard. Conversion is based on the weigh t percent of GVL converted, and selectivity was based on the weight fraction of each compound relative to the amount of GVL converted.