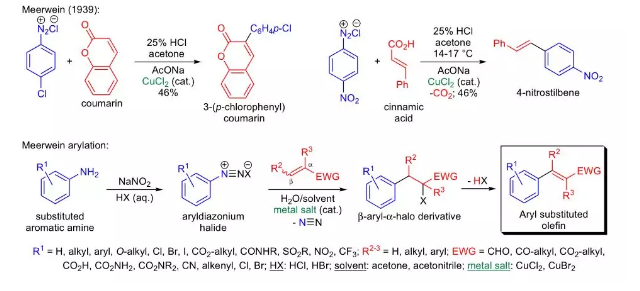
he arylation of substituted alkenes with aryldiazonium halides (formally the addition of an aryl halide to a carbon-carbon double bond) in the presence of a metal salt catalyst is known as theMeerwein arylation.
The general features of this reaction are: 1) the procedure is simple; no special laboratory equipment is needed; 2) the aryldiazonium halides are prepared by the diazotization of aromatic amines using sodium nitrite and aqueous hydrohalic acids and are not isolated, rather immediately reacted with the alkenes in the presence of an organic solvent (e.g., acetone, acetonitrile); 3) the presence of electron-withdrawing substituents on the aromatic ring tends to increase the yield, whereas electron-donating groups often give lower yields; 4) the alkene component usually has an electron-withdrawing substituent and mostly α,β- unsaturated carbonyl compounds are used; 5) if there are two electron-withdrawing substituents on the double bond, and they are attached to the same carbon and then the aryl group will add to the other sp 2 hydbridized carbon atom; 6) when each of the olefin carbon atoms has an electron-withdrawing substituent, regioisomeric products may be formed; however, the major product will arise from the most resonance stabilized radical intermediate; 7) cinnamic acids and maleic acids are arylated at the α-carbon, and the reaction is accompanied by decarboxylation which is a pH-dependent process; 8) alkynes with electron-withdrawing substituents also react, but the yields are often poor; 9) furan derivatives are arylated with ease under the reaction conditions; and 10) the initial product of the reaction is a substitution product (alkyl halide), which can be dehydrohalogenated under basic conditions to afford the corresponding aryl substituted olefin. The Meerwein arylation is not free of side reactions (e.g., Sandmeyer reaction, formation of azo compounds, etc.), which are the primary cause of the often moderate product yields.
Mechanism:
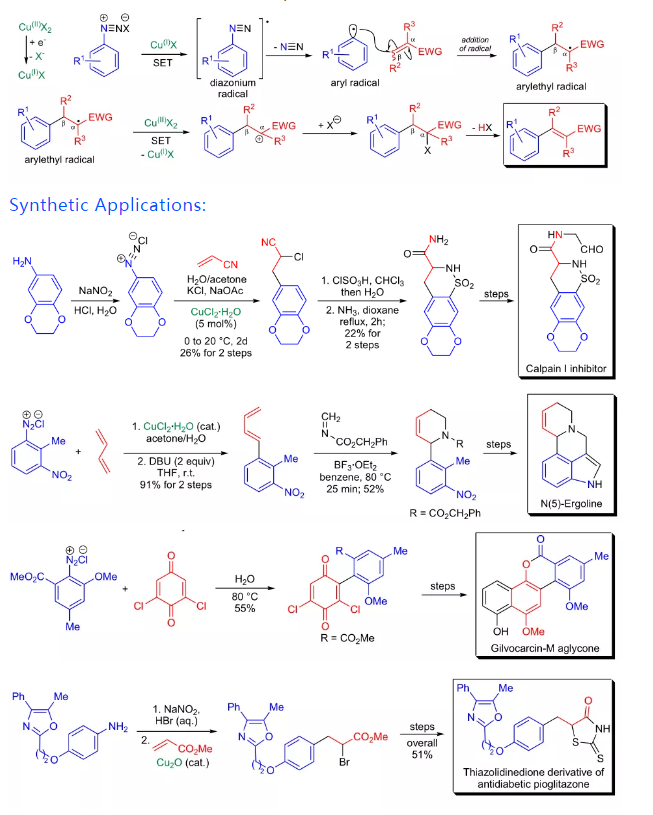
The mechanism of the Meerwein arylation is not completely understood. In his seminal paper, Meerwein proposed the involvement of aryl cations, however, this hypothesis was soon eliminated when J.K. Kochi suggested that aryl radicals are formed under the reaction conditions. The actual catalyst is a copper(I) species, which is formed in situ from copper(II) salts and carbonyl compounds (e.g., acetone which is often used as a solvent).
Reference:
1. Meerwein, H., Buchner, E., van Emster, K. Reaction of aromatic diazo compounds upon α,β-unsaturated carbonyl compounds. J. Prakt. Chem. 1939, 152, 237-266.
2. Rondestvedt, C. S., Jr. Arylation of unsaturated compounds by diazonium salts (the Meerwein arylation reaction). Org. React. 1960, 11,189-260.
3. Rondestvedt, C. S., Jr. Arylation of unsaturated compounds by diazonium salts (the Meerwein arylation reaction). Org. React. 1976, 24,225-259.
4. Galli, C. Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical. Chem. Rev. 1988, 88, 765-792.
5. Weis, C. D. Meerwein arylation reactions of olefins with anthraquinone diazonium hydrogen sulfates: formation of new carbon bonds at the carbon atoms C-1 and at C-1,5 of the anthraquinone system. Dyes Pigm. 1988, 9, 1-20.
6. Curran, D. P. Radical Addition Reactions. in Comp. Org. Synth. (eds. Trost, B. M.,Fleming, I.), 4, 715-777 (Pergamon, Oxford, 1991).
7. Minisci, F., Fontana, F., Vismara, E. Polar and enthalpic effects in free-radical reactions. Free-radical diazo coupling and reactivity of
carbohydrate radicals. Gazz. Chim. Ital. 1993, 123, 9-18.
8. Truce, W. E., Breiter, J. J., Tracy, J. E. The Meerwein arylation of vinyl sulfones. J. Org. Chem. 1964, 29, 3009-3014.
9. Filler, R., White, A. B., Taqui-Khan, B., Gorelic, L. Synthesis of aromatic α-amino acids via Meerwein arylation. Can. J. Chem. 1967, 45, 329-331.
10. Doyle, M. P., Siegfried, B., Elliott, R. C., Dellaria, J. F., Jr. Alkyl nitrite-metal halide deamination reactions. 3. Arylation of olefinic
compounds in the deamination of arylamines by alkyl nitrites and copper(II) halides. A convenient and effective variation of the Meerwein arylation reaction. J. Org. Chem. 1977, 42, 2431-2436.