Product name:4,4-Difluorocyclohexanecarboxylic acid
MF:C7H10F2O2
Cas No.:122665-97-8
MW:164.15
Structure: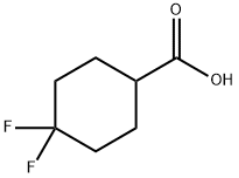
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
potassium hydroxide; ethanol; water;
Reactants can be synthesized in 1 step.
Wang, Ying-Kai, US2013/183269, A1, (2013) Evaporation of the solvent afforded ethyl 4,4-difluoro-1-methylcyclohexanecarboxylate (222 mg) as a light brown oil which was dissolved in EtOH and was added a solution of KOH (112 mg, 2.000 mmol) in water (2.00 mL). The reaction mixture was heated to reflux overnight, then cooled and acidified and extracted with EtOAc to afford Cap-5 as a light brown solid. 1H NMR (400 MHz, CHLOROFORM-d) delta 2. 26-2.17 (m, 2H), 2.07-1.95 (m, 2H), 1.94-1.83 (m, 2H), 1.63-1.54 (m, 2H), 1.31 (s, 3H).