Product name:5-Methylbenzo[b]thiophene
MF:C9H8S
Cas No.:14315-14-1
MW:148.22
Structure: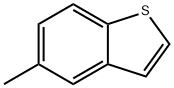
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
sodium hydride; phosphorous pentoxide; phosphoric acid; tetrahydrofuran; mineral oil;
Reactants are commercially availlable.
Iwakoshi, Mitsuhiko, US2015/282482, A1, (2015) 12116] Step 112117] A mixture of 5.2 g ofbromoacetaldehyde diethylacetal and 10 ml of tetrahydrofuran was added to a mixture of 4.00 g of 4-methylbenzenethiol, 1.4 g of 60percent sodium hydride, and 3 5 ml of tetrahydroffiran. The reaction mixture was stirred for 15 hours at room temperature. Ten (10) ml of aqueous saturated ammonium chloride solution was added to the reaction mixture, and extracti on was performed three times by using tert-butyl methyl ether. The collected organic layer was washed with water and saturated saline, dried over magnesium sulfate, and then concentrated under reduced pressure. The residues were added to a mixture of 5 g of diphosphorus pentoxide and lOg ofphosphori c acid that had been stirred for 45 minutes at 175°C., and the residue was stirred for 5 minutes. T he reaction mixture was poured into ice water, and extraction was performed three times by using tert-butyl methyl ethet The collected organic layer was washed with water and saturated saline, dried o ver magnesium sulfate, and then concentrated under reduced pressure. The residues were subjected to silica gel colunm chromatography, thereby obtaining 2.77 g of 5-methylbenzo[b] thiophene