中文名称:2-氟丙烯酸
英文名称:2-FLUOROACRYLIC ACID
分子式:C3H3FO2
Cas No.:430-99-9
分子量:90.05
结构式: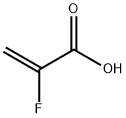
规格:98%
外观:白色固体
用途:基础原料、有机中间体、医药中间体
合成工艺如下:
1-(chloromethyl)-4-fluoro-1,4-diazoniabicyclo-[2.2.2]octane bis(tetrafluoroborate); water;
Reactants are commercially availlable.
Alem, Naziha, US2015/158872, A1, (2015) [0456] 1,1,3,3-tetramethoxypropane 35a (20 g, 121.8mmol) was dissolved in water (200 ml). p-Toluenes ulphonicacid monohydrate (23.17 g, 121.8 mmol) was added and themixture stirred at 19-20°C. for 90 minutes.
1-(Chloromethyl)-4-fluoro-1 ,4-diazoniabicyclo[2.2.2]octane ditetrafluoroborate37 (Selectfluor, 1.4 eqv, 60.4 g, 170.5 mmol)was added portionwise. The addition was endothermic (20.1 oC. to 19 .4°C.) however the temperature began to rise slowlyonce the addition was complete (temp increased t o 25.4°C.over 45 minutes). The selectfluor dissolved over 1 hr. Themixture was allowed to stir at a mbient temperature for 18 hrs.The mixture was homogeneous after this time. DMSO (150ml) was added sl owly over 5 minutes. The addition wasexothermic-the temperature increased from 20.4°C. to34.2°C. d uring the addition. The mixture then began to cool.The resulting mixture was stirred for 45 minutes. Compound3 (21.4 g, 115.7 mmol) was then added portionwise. Theaddition was not exothermic. The mixt ure was heated to 85°C. for 4 hrs (Lc/Ms profile was identical at 2 hr and 4 hr timepoints). The sti rred mixture was then allowed to cool to ambienttemperature overnight. The resulting reaction mixtur ewas a slurry. Water (150 ml) was added slowly to the resultingslurry. The temperature increased fro m 20.4°C. to 21.5°C.The slurry was stirred for 2 hrs, and then the product wasisolated by filtrati on. The cake was washed with water anddried on the sinter to a beige solid (15.5 g). The product was further dried in a vac oven at 40°C. for 20 hrs. This gavecompound 4a as a beige solid (13.5 g, 50p ercent yield). HPLCpurity 97.7percent area; 1H NMR (500 MHz, DMSO-d6) o 4.83(2H, d), 5.29 (lH, d), 5.49 (lH, d), 6.04-6.14 (lH, m), 6.57(2H, brs), 8.80 (lH, m), 9.40 (lH, m); 19F NMR (500 MHz,DMSO-d6) o -153.1.