Product name:(5-Methoxypyridine-2-yl)methanol
MF:C7H9NO2
Cas No.:127978-70-5
MW:139.15
Structure: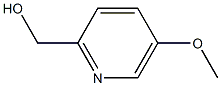
Purity:98%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
With water; potassium hydroxide In methanol for 2 h; Reflux
Reactants are commercially availlable.
Add 15ml water and 2G sodium hydroxide into a 100ml eggplant bottle, stir and dissolve, add 35ml ethanol, and then add 5g 2-chloro-3,3-difluoro-1-cyclobutyric acid (6). The system is to purge three times with nitrogen and add 100mg 10% palladium carbon. The reaction was heated to 40 ℃ and stirred in hydrogen atmosphere for 12 hours. The HPLC products disappeared completely and the reaction was terminated. Reduce the temperature to room temperature, filter to remove the carbon supported palladium, and wash the filter cake twice with 10ml ethanol. The filtrate was distilled at 35 ℃. The ethanol was removed at ° C. The remaining water phase was diluted to about 0-5 with 15ml ice water, adjusted pH = 1-2 with 5% hydrochloric acid, extracted twice with ethyl acetate (30mlx2), combined with organic phase, dried with anhydrous sodium sulfate, and distilled under reduced pressure. 4 g of crude product was obtained at 35 ℃, and 3.9 g of white product 3,3-difluorocyclobutyric acid (1) was obtained with 98% yield