Product name:1-BOC-3,4-epoxypiperidine
MF:C10H17NO3
Cas No.:161157-50-2
MW:199.25
Structure: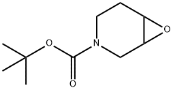
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
sodium hydrogencarbonate; dichloromethane; water;
Reactants are commercially availlable.
Fujisawa Pharmaceutical Co., Ltd., US6355640, B1, (2002) To a solution of 1-tert-butoxycarbonyl-1,2,3,6-tetrahydropyridine (18.9 g) in dichloromethane (400 ml).. was added in turn sodium hydrogen carbonate (11.3 g) and m-chloroperoxybenzoic acid (23.4 g) wi th care at 0°C., and which was stirred for 2 hours.. Insoluble material was removed by filtration, mother liquor was washed saturated sodium chloride in water (100 ml) and dried over magnesium sulfat e.. Evaporation of the solvent gave a residue, which was dissolved in n-hexane (300 ml) and insoluble material was removed by filtration.. Mother liquor was concentrated in vacuo, the remainings were dissolved in ethyl acetate (300 ml) and washed in turn with saturated sodium hydrogen carbonate in water (100 ml*5) and saturated sodium chloride in water (100 ml*2), which was dried over magnesium su lfate.. Evaporation of the solvent gave a residue, which was chromatographed on silica gel (350 ml) eluding in turn with 10percent, 20percent and 30percent ethyl acetate in n-hexane.. Tractions, conta ining desired product, were collected and concentrated in vacuo to give 1-tert-butoxycarbonyl-3,4-epoxypiperidine (13.7 g). NMR (CDCl3, delta): 1.45 (9H, s), 1.75-2.10 (2H, m), 3.00-4.00 (6H, m).