Product name:4-(Piperidin-4-yl)Morpholine
MF:C9H18N2O
Cas No.:53617-35-9
MW:170.25
Structure: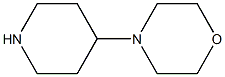
Purity:95%
Appearance:white solid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
potassium hydroxide; sodium borohydride; toluene-4-sulfonic acid; ethanol; toluene;
Reactants are commercially availlable.
Chiba, Kenji, US2003/203909, A1, (2003) STARTING MATERIAL SYNTHETIC EXAMPLE 10 4-morpholinopiperidine 1-Ethoxycarbonyl-4-piperidone (250 g), morpholine (152.0 g) and p-toluenesulfonic acid (5 g) were added to toluene (600 ml) and the mixtur e was heated under reflux for 15 hr with dehydration. Then, the solvent was evaporated and the obtained oily component was added to a suspension of ethanol (1L) containing sodium borohydride (58 g) un der ice-cooling. The mixture was further stirred at room temperature for 10 hr. The reaction mixture was treated with diluted hydrochloric acid and the solvent was evaporated under reduced pressure. Thereto were added 2-propanol (2 L) and potassium hydroxide (365 g) and the mixture was heated under reflux at 80°C. for 15 hr. The solvent was evaporated under reduced pressure. The obtained oily c omponent was purified by distillation to give the title compound (168 g). boiling point: 95-100°C./ 1.2 mmHg 1H-NMR(400 MHz, CDCl3)delta(ppm): 1.3-1.4 (2H, m), 1.8-1.9 (2H, m), 2.2-2.3 (1H, m), 2.5-2. 6 (4H, m), 3.1-3.2 (2H, m), 3.7-3.8 (4H, m)