Product name:N-FORMYL-L-LEUCINE
MF:C7H13NO3
MW: 159.18
Cas No.:6113-61-7
Structure: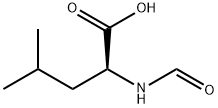
Appearance: White solid
Purity:98%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthetic route is as follows:
glyceroacetonide-oxyma; sodium hydrogencarbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; water;
Reactants are commercially availlable.
Tetrahedron Letters, vol. 54, 16, (2013), p. 2077 - 2081 General procedure: To a solution of amine (1 equiv), formic acid (5 equiv), sodium bicarbonate (10 equiv), and glyceroacetonide-Oxyma 1 (2 equiv) in H2O (0.2?0.3 M) solution was added EDCI (2 equiv) The reaction mixture was stirred for 3 h and quenched with 1percent aq HCl. The aqueous phase was extracted with EtOAc (or CHCl3 or CHCl3?MeOH (10/1). The combined organic extracts were dried over Na2SO4 and evaporated in vacuo. Purification by a silica gel chromatography (or seph adex LH20) afforded the desired compound (yields were given in Table 1). Similarly, N-formylations were performed with Oxyma 1 in DMF?H2O (9/1).