Norris Pharm sells well:
Product name:2-METHYL-3,1-BENZOXAZA-4-ONE
MF:C9H7NO2
Cas No.:525-76-8
MW:107.58
Structure: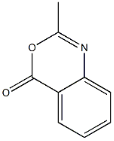
Purity:95%
Appearance:pale yellow to brown solid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
methanol;
Reactants are commercially availlable.
Easy and efficient strategy was undertaken to synthesize the target quinazoline Schiff?s base which involves the following three steps. In the first synthesis step, a warm solution of methyl anthranil ate reacts with acetic anhydride in 20mL methanol, results in the formation of a cyclic compound 2-methyl-4H-benzo [d] [1,3] oxazin-4-one. The second step involves the reaction between 2-methyl-4H-ben zo [d] [1,3] oxazin-4-one and hydrazine hydrate in 20mL hot methanol to afford 3-amino-2-methylquinazoline-4(3H)-one [18]. Finally, in the third step the simple condensation reaction between 3-amino-2 -methyl quinazoline-4-one and 2-hydroxy-1-naphthaldehyde results in the formation of the Schiff?s base (E)-3-((2-hydroxynaphthalen-1-yl) methyleneamino)-2-methylquinazoline-4(3H)-one HNMAMQ as present ed in supplementary file Fig. S1. The progress of the reaction was continuously monitored by the aid of thin layer chromatography (TLC) on a silica gel-G coated aluminum plates (Merck) and spots were visualized by exposing the dry plates to iodine vapors.