Norris Pharm sells well:
Product name:Azidoacetic acid
MF:C2H3N3O2
Cas No.:18523-48-3
MW:107.58
Structure: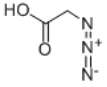
Purity:95%
Appearance:yellow liquid
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
sodium azide; dimethyl sulfoxide;
Reactants are commercially availlable.
GEORG-AUGUST-UNIVERSITAet GOeTTINGEN STIFTUNG OeFFENTLICHEN RECHTS (OHNE BEREICH HUMANMEDIZIN), WO2007/89149, A2, (2007) NaN3 (22.2 g, 341 mmol) was added slowly to a solution of methyl chloroacetate (20.0 mL, 227 mmol) in DMSO (100 mL). After stirring at room temperature for 24 h, water (150 mL) was added and the mixtu re was extracted with Et2O (3 x 100 mL). The combined organic fractions were dried (MgSO^ and concentrated in vacuo to 50 mL. Then, a solution of aldehyde 15 (5.37 g, 25.0 mmol) in MeOH (50 mL) was ad ded and the mixture was cooled to -30 0C. After that, the reaction mixture was treated with 5.4 M Na OMe/MeOH (35.0 mL, 189 mmol) within 30 min at -30 °C, warmed to 0 0C and diluted with MeOH (50 mL). After stirring for 16 h at 0 0C, water (200 mL) was added and the mixture was extracted with CH2Cl2 (3 x 200 mL). The combined organic fractions were washed with brine (200 mL), dried (MgSO4), and the solvent was removed in vacuo to give 16 (6.84 g, 88percent from 15) as a pale yellow solid that was used for the next step without further purification. 1H NMR (300 MHz, CDCl3): delta = 3.8 4 (t, J= 6.1 Hz, 2 H, 2"-H2), 3.87, 3.88 (2 x s, 6 H, OMe, CO2Me), 4.29 (t, J= 6.1 Hz, 2 H, 1"-H2), 6.83 (s, 1 H, V-R), 6.87 (d, J= 8.5 Hz, 1 H, 5-H), 7.36 (dd, J= 8.5, 2.1 Hz, 1 H, 6-H), 7.53 (d, J= 2.1 Hz, 1 H, 2-H) ppm; 13C NMR (50.3 MHz, CDCl3): delta = 41.6 (C-2"), 52.8 (CO2Me), 56.0 (OMe), 69.3 (C-I"), 111.5 (C-2), 116.2 (C-5), 123.4 (C-21), 125.4, 126.0, 126.2 (C-I, C- 1', C-6), 147.1, 150. 9 (C-3, C-4), 164.1 (C=O) ppm.