1) 用 TosMIC 直接从酮转化为氰基
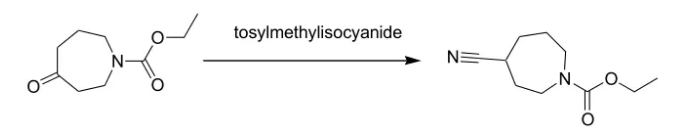
To a 250 mL round-bottomed flask equipped with condenser and nitrogen inlet were added 4.34 g (23.49 mmol) N-carboethoxyperhydroazepin-4-one (prepared according to the procedure given by Z. G. Finney and T. N. Riley, J. Med. Chem., 23, 895, 1980), 10.53 g (54.02 mmol) tosylmethylisocyanide and 117 mL 1,2-dimethoxyethane. The solution was cooled to 0 deg C and 2.48 mL (54.02 mmol) ethanol and 9.21 g (82.2 mmol) potassium t-butoxide were added. The mixture was heated at 60 deg C for 18 hours, cooled and concentrated. The residue was taken up in ethyl acetate, washed with brine, dried over sodium sulfate and concentrated to give an oil. The oil was purified by chromatography on silica gel using hexane/ethyl acetate as eluent to afford 4.6 g (100percent) of oil.
Reference: 72800; Patent; Pfizer Inc.; Publ.: US5373003 A1 (1994/12/13)
2)用2,4,6- 三异丙基磺酰肼-KCN
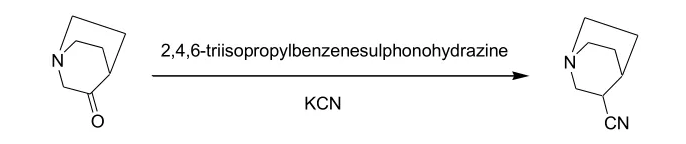
3-Quinuclidinone (24.2 g, 0.19 mol) and 2,4,6-triisopropylbenzenesulphonohydrazide (72 g, 0.24 mol) were stirred together in anhydrous MeOH (250 mL) for 3 h. Potassium cyanide (33.8 g, 0.51 mol) was added and the mixture was heated under reflux for 5 h. The residue after evaporation of the solvent was partitioned between water and CH2C12. The organic phase was dried and evaporated and the residue was fractionally distilled under reduced pressure to give 3-cyanoquinuclidine (32, 6.1 g).
Reference: J. Med. Chem. 1990, 33, 1128-1138