Product name:4-((N-BOC-AMINO)METHYL)PHENYLBORONIC ACID
MF:C12H18BNO4
Cas No.:489446-42-6
MW:251.09
Structure: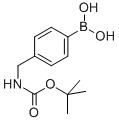
Purity:95%
Product use:Basic raw materials, organic intermediates, pharmaceutical intermediates
The synthesis process is as follows:
caesium carbonate; DME; water;
Reactants are commercially availlable.
WATANABE, Hiroyuki, WO2015/161142, A1, (2015) Boc20 (178 uL, 0.77 mmol) was added to a solution of (4-(aminomethyl)phenyl)boronic acid hydrochloride (143 mg, 0.77 mmol) and Cs2C03 (665 mg, 2.04 mmol) in DME (10 mL) and water (1.0 mL) at rt. After being stirred at rt for 30 min, Suzuki coupling was carried out as follow: 2-chloro-N-(3- (lH-imidazol-l-yl)propyl)-3-phenylquinoxaline-6-carboxamide (201 mg, 0.54 mmol) (prepared according to Exampl e 3, step 7) and Pd(dppf)Cl2? CH2C12 (42.0 mg, 51.4 muiotaetaomicron) were added to the reaction mix ture. The mixture was heated at 100 °C for 10 h under microwave irradiation. After cooling, the mixt ure was poured into saturated aqueous solution of NaHC03 and extracted with EtOAc. The organic layer was separated, washed with water and brine, dried over MgS04 and concentrated in vacuo. The residue was purified by NH silica gel chromatography to give teri-butyl [4-(6-{[3-(lH-imidazol-l- yl)propyl]carbamoyl}-3-phenylquinoxalin-2-yl)benzyl]carbamate (187 mg, 65percent, H NMR (300 MHz, DMSO-i/6) delta 1.23-1.52 (m, 9 H), 2.00-2.12 (m, 2 H), 3.32-3.39 (m, 2 H), 4.05^1.19 (m, 4 H), 6.91 (s, 1 H), 7.10-7.30 (m, 3 H), 7.32-7.77 (m, 9 H), 8.12-8.38 (m, 2 H), 8.69 (d, J= 1.3 Hz, 1 H), 8.91 (t, J= 5 .2 Hz, 1 H)